Abstract
Introduction: LR-MDS comprise a heterogeneous group of clonal myeloid neoplasms characterized by ineffective hematopoiesis, dysplasia(s), cytopenia(s), and relatively good-risk karyotypic and molecular abnormalities. The primary therapeutic strategy for pts with symptomatic LR-MDS is supportive care with red blood cell (RBC)/platelet transfusions and growth factor support such as treatment with ESAs. However, length of treatment and clinical outcomes vary among pts and are poorly understood in real-world clinical practice, particularly in the context of current guidelines. This medical chart review explores the impact of clinical characteristics at diagnosis on clinical outcomes of pts with LR-MDS treated primarily in community settings.
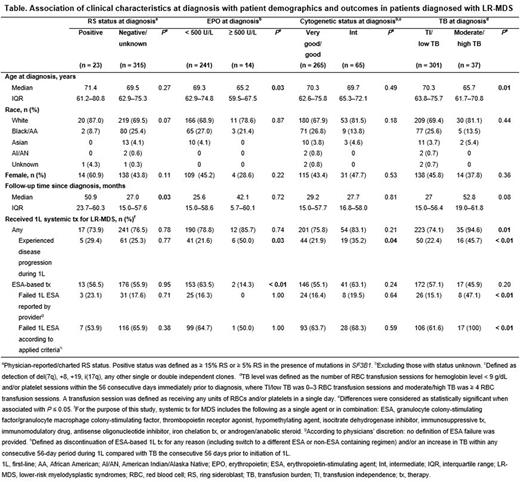
Methods: This was a retrospective, observational, US multisite chart review of adult pts diagnosed with LR-MDS between January 1, 2015 and June 24, 2020. Eligible pts had ≥ 1 year of follow-up after diagnosis unless follow-up was < 1 year due to death. Pts who participated in a clinical trial were excluded. Demographic characteristics assessed included age at diagnosis, sex, and race/ethnicity. Four sets of subgroups were analyzed based on the following clinical characteristics collected at diagnosis: ring sideroblast (RS) status; erythropoietin (EPO) level; cytogenetic risk status; and transfusion burden (TB) level. TB level was defined as the number of RBC transfusion sessions for hemoglobin level < 9 g/dL and/or platelet sessions within the 56 consecutive days immediately prior to diagnosis, where transfusion independence (TI)/low TB was 0-3 RBC transfusion sessions and moderate/high TB was ≥ 4 RBC transfusion sessions. Treatment outcomes included physician-reported disease progression or ESA failure. ESA failure was determined per physician reports and/or calculated based on pre-specified criteria, which included discontinuation or switch of ESA-based therapy and/or increased TB within any consecutive 56-day period during ESA-based treatment. Statistical comparisons of characteristics and outcomes between subgroups were conducted using Fisher's exact or Chi-square tests for categorical data, Wilcoxon tests for continuous data, and log rank tests for Kaplan-Meier estimates. Statistical significance was defined as P ≤ 0.05.
Results: Among 338 pts with LR-MDS identified, 23 (6.8%) were RS+, 14 (4.1%) had EPO levels ≥ 500 U/L, 65 (19.2%) had intermediate cytogenetics, and 37 (10.9%) had moderate/high TB at diagnosis. Median age at diagnosis was lower in pts with EPO levels ≥ 500 U/L vs < 500 U/L and in those with moderate/high TB vs TI/low TB. There were no statistically significant differences in race or sex among subgroups. The median follow-up time since diagnosis was longer for pts with RS+ status (50.9 months) vs those with negative/unknown RS status (27.0 months). No statistically significant differences in follow-up time were observed for the other subgroups (Table).
A greater proportion of pts with moderate/high TB (94.6%) vs TI/low TB (74.1%) received any first-line (1L) systemic therapy for LR-MDS; no significant differences were observed for the other subgroups. Among those who received any 1L systemic therapy, a greater proportion of pts with disease progression at 1L was observed for those with EPO levels ≥ 500 U/L (50.0%) vs < 500 U/L (21.6%), intermediate (35.2%) vs good/very good (21.9%) cytogenetics, and moderate/high TB (45.7%) vs TI/low TB (22.4%) at diagnosis (Table).
A smaller proportion of pts with EPO levels ≥ 500 U/L (14.3%) vs < 500 U/L (63.5%) at diagnosis received an ESA-based therapy at 1L. Among pts who received 1L ESA-based therapy, a greater proportion of pts with moderate/high TB (100%) vs TI/low TB (61.6%) experienced ESA failure. No statistically significant differences in 1L ESA failure were observed for the other subgroups (Table).
Conclusions: This real-world medical chart review study in the US community care setting suggests that among pts with LR-MDS who receive 1L systemic therapy, more pts who have EPO levels ≥ 500 U/L, intermediate cytogenetics, and/or moderate/high TB at diagnosis may experience disease progression during treatment than those who do not. These results are in line with previous findings that EPO levels, cytogenetic status, and TB at diagnosis may be important predictors of 1L treatment outcomes. Additional studies with larger sample sizes may be useful to further explore these findings.
Disclosures
Mukherjee:Aplastic Anemia and MDS International Foundation: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Celgene/Acceleron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Partnership for Health Analytic Research, LLC: Honoraria; Eusa Pharma: Consultancy, Other: Advisor or review panel participant; Teaching and Speaking; Jazz Pharmaceuticals: Other: Principal investigator for Investigator Initiated Trials (the Institution gets the funding), Research Funding; McGraw Hill Hematology Oncology Board Review: Honoraria, Other: Advisor or review panel participant; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; BioPharm: Consultancy. Savill:Astra Zeneca: Consultancy; Cardinal Health: Current Employment, Current holder of stock options in a privately-held company; Genentech: Ended employment in the past 24 months; Pfizer: Consultancy; Daiichi Sankyo: Consultancy; Incyte: Consultancy; Morphosys: Consultancy; Janssen: Consultancy; Bayer: Consultancy. Gajra:Cellectar: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Cardinal Health Specialty Solutions: Consultancy, Current Employment, Current equity holder in publicly-traded company. Gentile:Cardinal Health: Current Employment. Falkenstein:Cardinal Health Specialty Solutions: Current Employment. Miller:Cardinal Health: Current Employment. Laney:Cardinal Health: Current Employment. McBride:BMS: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal